Revisiting prior families for trees: Part II
Jason Willwerscheid
7/22/2020
Last updated: 2020-07-23
Checks: 6 0
Knit directory: drift-workflow/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.2.0). The Report tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20190211) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: docs/.DS_Store
Ignored: docs/assets/.DS_Store
Ignored: output/
Untracked files:
Untracked: analysis/extrapolate3.Rmd
Untracked: analysis/extrapolate4.Rmd
Unstaged changes:
Modified: drift-workflow.Rproj
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 2af9954 | Jason Willwerscheid | 2020-07-23 | workflowr::wflow_publish(“analysis/pm1_priors2.Rmd”) |
suppressMessages({
library(flashier)
library(drift.alpha)
library(tidyverse)
})I continue to use the four-population tree that I’ve been using, but I change the admixture proportions for Population \(E\) to 60% \(B\), 25% \(C\), and 15% \(D\). I did so because I wanted to make the problem slightly less nice for the approach I’m trying out here.
Namely, I’m initializing the tree by using a mixture of point masses at -1, 0, and +1, with the point mass at zero corresponding to either zero participation in the corresponding subtree or a 50-50 admixture. For example, admixture proportions of \(1/2\), \(1/3\), and \(1/6\) would give a 50-50 admixture for the top split (between Populations \(A/B\) and Populations \(C/D\)), so that we’d expect the loading for Population \(E\) to be zero. With a 60-25-15 admixture, we’d instead like to see a loading of \(1/5\).
After initializing the tree, I’ll re-fit using a more flexible mixture of point masses at -1 and +1 and then a uniform component on \([-0.9, 0.9]\). I don’t want to allow the uniform component to span the entire interval between -1 and +1: the optimization seems to be a little easier when the difference between the pointmass and the uniform component is exaggerated. This should be fine for all but the worst of cases (i.e., very small admixture proportions for the top split).
set.seed(666)
n_per_pop <- 60
p <- 10000
a <- rnorm(p)
b <- rnorm(p)
c <- rnorm(p)
d <- rnorm(p, sd = 0.5)
e <- rnorm(p, sd = 0.5)
f <- rnorm(p, sd = 0.5)
g <- rnorm(p, sd = 0.5)
popA <- c(rep(1, n_per_pop), rep(0, 4 * n_per_pop))
popB <- c(rep(0, n_per_pop), rep(1, n_per_pop), rep(0, 3 * n_per_pop))
popC <- c(rep(0, 2 * n_per_pop), rep(1, n_per_pop), rep(0, 2 * n_per_pop))
popD <- c(rep(0, 3 * n_per_pop), rep(1, n_per_pop), rep(0, n_per_pop))
popE <- c(rep(0, 4 * n_per_pop), rep(1, n_per_pop))
E.factor <- 0.60 * (a + b + e) + 0.25 * (a + c + f) + 0.15 * (a + c + g)
Y <- cbind(popA, popB, popC, popD, popE) %*%
rbind(a + b + d, a + b + e, a + c + f, a + c + g, E.factor)
Y <- Y + rnorm(5 * n_per_pop * p, sd = 0.1)
plot_dr <- function(dr) {
sd <- sqrt(dr$prior_s2)
L <- dr$EL
LDsqrt <- L %*% diag(sd)
K <- ncol(LDsqrt)
plot_loadings(LDsqrt[,1:K], rep(c("A", "B", "C", "D", "E"), each = n_per_pop)) +
scale_color_brewer(palette="Set2")
}
tree.fn = function(x, s, g_init, fix_g, output, ...) {
if (is.null(g_init)) {
g_init <- ashr::unimix(rep(1/3, 3), c(-1, 0, 1), c(-1, 0, 1))
}
return(flashier:::ebnm.nowarn(x = x,
s = s,
g_init = g_init,
fix_g = fix_g,
output = output,
prior_family = "ash",
prior = c(10, 1, 10),
...))
}
prior.tree = function(...) {
return(as.prior(sign = 0, ebnm.fn = function(x, s, g_init, fix_g, output) {
tree.fn(x, s, g_init, fix_g, output, ...)
}))
}
flextree.fn = function(x, s, g_init, fix_g, output, ...) {
if (is.null(g_init)) {
g_init <- ashr::unimix(rep(1/3, 3), c(-1, -0.9, 1), c(-1, 0.9, 1))
}
return(flashier:::ebnm.nowarn(x = x,
s = s,
g_init = g_init,
fix_g = fix_g,
output = output,
prior_family = "ash",
prior = c(10, 1, 10),
...))
}
prior.flextree = function(...) {
return(as.prior(sign = 0, ebnm.fn = function(x, s, g_init, fix_g, output) {
flextree.fn(x, s, g_init, fix_g, output, ...)
}))
}Here’s what the initial tree looks like with the three-pointmass priors. I added extra “mean” factors for Populations \(A/B\) and Populations \(C/D\) (Factors 3 and 5 below), basically to subtract out the contribution from Population \(E\) before splitting. I’ll be able to remove those factors when I use the more relaxed priors.
ones <- matrix(1, nrow = nrow(Y), ncol = 1)
ls.soln <- t(solve(crossprod(ones), crossprod(ones, Y)))
fl <- flash.init(Y) %>%
flash.set.verbose(0) %>%
flash.init.factors(EF = list(ones, ls.soln),
prior.family = c(prior.tree(), prior.normal())) %>%
flash.fix.loadings(kset = 1, mode = 1L) %>%
flash.backfit() %>%
flash.add.greedy(prior.family = c(prior.tree(), prior.normal())) %>%
flash.backfit()
set1 <- (fl$flash.fit$EF[[1]][, 2] < -0.9)
set2 <- (fl$flash.fit$EF[[1]][, 2] > 0.9)
set3 <- !set1 & !set2 # admixed individuals
# Add a mean factor for Populations A/B.
next.factor <- matrix(1L * set1, ncol = 1)
ls.soln <- t(solve(crossprod(next.factor), crossprod(next.factor, Y)))
fl2 <- fl %>%
flash.init.factors(EF = list(next.factor, ls.soln),
prior.family = c(prior.tree(), prior.normal())) %>%
flash.fix.loadings(kset = 3, mode = 1L) %>%
flash.backfit(3)
# Split A/B.
next.factor <- matrix(sample(c(-1, 1), size = nrow(Y), replace = TRUE) * set1, ncol = 1)
ls.soln <- t(solve(crossprod(next.factor), crossprod(next.factor, Y)))
fl2 <- fl2 %>%
flash.init.factors(EF = list(next.factor, ls.soln),
prior.family = c(prior.tree(), prior.normal())) %>%
flash.fix.loadings(kset = 4, mode = 1L, is.fixed = set2 | set3) %>%
flash.backfit(4)
# Add a mean factor for Populations C/D.
next.factor <- matrix(1L * set2, ncol = 1)
ls.soln <- t(solve(crossprod(next.factor), crossprod(next.factor, Y)))
fl2 <- fl2 %>%
flash.init.factors(EF = list(next.factor, ls.soln),
prior.family = c(prior.tree(), prior.normal())) %>%
flash.fix.loadings(kset = 5, mode = 1L) %>%
flash.backfit(5)
# Split C/D.
next.factor <- matrix(sample(c(-1, 1), size = nrow(Y), replace = TRUE) * set2, ncol = 1)
ls.soln <- t(solve(crossprod(next.factor), crossprod(next.factor, Y)))
fl2 <- fl2 %>%
flash.init.factors(EF = list(next.factor, ls.soln),
prior.family = c(prior.tree(), prior.normal())) %>%
flash.fix.loadings(kset = 6, mode = 1L, is.fixed = set1 | set3) %>%
flash.backfit(6)
plot_dr(init_from_flash(fl2))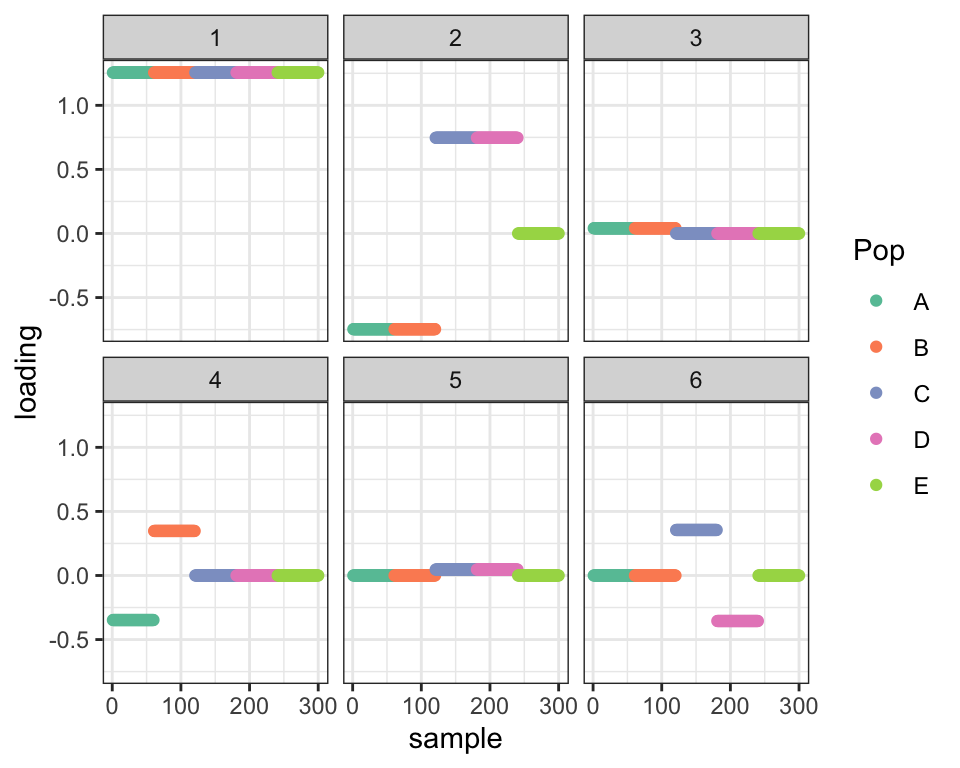
Here’s what the fit looks like after relaxation.
# Remove the redundant "mean" factors.
fl3 <- fl2 %>%
flash.fix.loadings(kset = 4, mode = 1L, is.fixed = set2) %>%
flash.fix.loadings(kset = 6, mode = 1L, is.fixed = set1) %>%
flash.remove.factors(c(3, 5)) %>%
flash.backfit()
# Relax the priors.
for (k in 1:4) {
fl3$flash.fit$ebnm.fn[[k]][[1]] <- flextree.fn
}
# Refit.
fl3 <- fl3 %>% flash.backfit(warmstart = FALSE)
plot_dr(init_from_flash(fl3))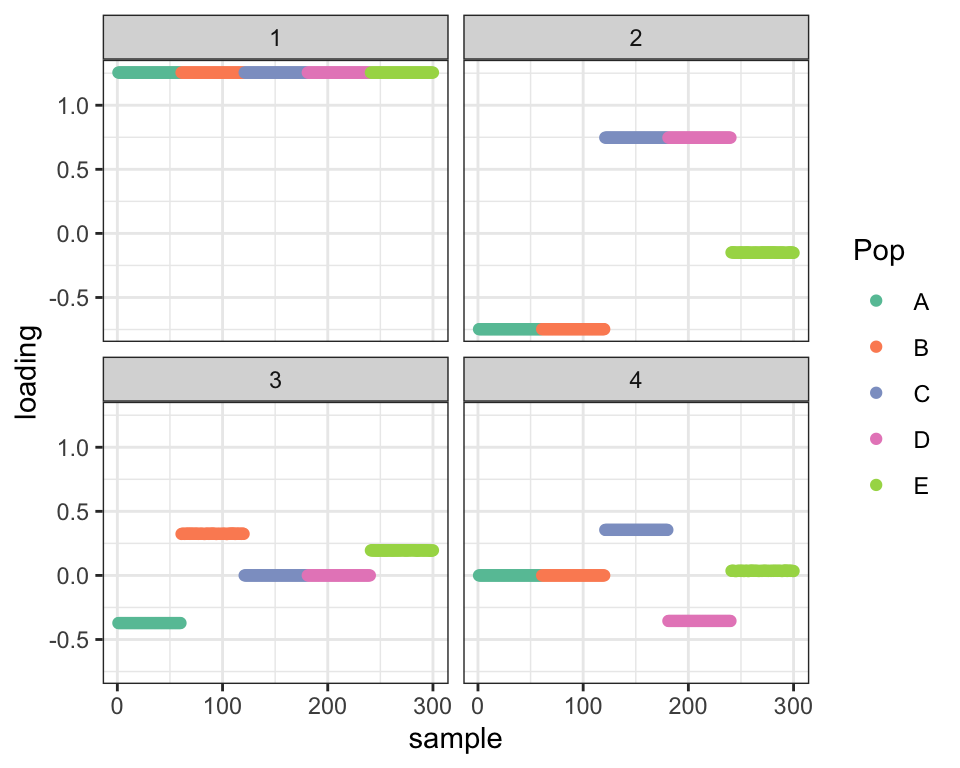
This looks pretty good. For Population \(E\), we want to see loadings of \(0.4 - 0.6 = -0.2\) for the top split (Factor 2), \(0.6\) for Factor 3, and \(0.25 - 0.15 = 0.1\) for Factor 4. And that’s pretty much what we see (with the exception that there’s noticeable shrinkage for Factor 3):
LL <- colMeans(fl3$flash.fit$EF[[1]][241:300, ])
names(LL) <- paste("Factor", 1:4)
round(LL, 2)#> Factor 1 Factor 2 Factor 3 Factor 4
#> 1.00 -0.20 0.52 0.10
sessionInfo()#> R version 3.5.3 (2019-03-11)
#> Platform: x86_64-apple-darwin15.6.0 (64-bit)
#> Running under: macOS Mojave 10.14.6
#>
#> Matrix products: default
#> BLAS: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRblas.0.dylib
#> LAPACK: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRlapack.dylib
#>
#> locale:
#> [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
#>
#> attached base packages:
#> [1] stats graphics grDevices utils datasets methods base
#>
#> other attached packages:
#> [1] forcats_0.4.0 stringr_1.4.0 dplyr_0.8.0.1
#> [4] purrr_0.3.2 readr_1.3.1 tidyr_0.8.3
#> [7] tibble_2.1.1 ggplot2_3.2.0 tidyverse_1.2.1
#> [10] drift.alpha_0.0.9 flashier_0.2.5
#>
#> loaded via a namespace (and not attached):
#> [1] Rcpp_1.0.4.6 lubridate_1.7.4 invgamma_1.1
#> [4] lattice_0.20-38 assertthat_0.2.1 rprojroot_1.3-2
#> [7] digest_0.6.18 truncnorm_1.0-8 R6_2.4.0
#> [10] cellranger_1.1.0 plyr_1.8.4 backports_1.1.3
#> [13] evaluate_0.13 httr_1.4.0 pillar_1.3.1
#> [16] rlang_0.4.2 lazyeval_0.2.2 readxl_1.3.1
#> [19] rstudioapi_0.10 ebnm_0.1-21 irlba_2.3.3
#> [22] whisker_0.3-2 Matrix_1.2-15 rmarkdown_1.12
#> [25] labeling_0.3 munsell_0.5.0 mixsqp_0.3-40
#> [28] broom_0.5.1 compiler_3.5.3 modelr_0.1.5
#> [31] xfun_0.6 pkgconfig_2.0.2 SQUAREM_2017.10-1
#> [34] htmltools_0.3.6 tidyselect_0.2.5 workflowr_1.2.0
#> [37] withr_2.1.2 crayon_1.3.4 grid_3.5.3
#> [40] nlme_3.1-137 jsonlite_1.6 gtable_0.3.0
#> [43] git2r_0.25.2 magrittr_1.5 scales_1.0.0
#> [46] cli_1.1.0 stringi_1.4.3 reshape2_1.4.3
#> [49] fs_1.2.7 xml2_1.2.0 generics_0.0.2
#> [52] RColorBrewer_1.1-2 tools_3.5.3 glue_1.3.1
#> [55] hms_0.4.2 parallel_3.5.3 yaml_2.2.0
#> [58] colorspace_1.4-1 ashr_2.2-51 rvest_0.3.4
#> [61] knitr_1.22 haven_2.1.1